Introduction
Polymers are widely found in plastics. Many products from the chemical industry are synthetic polymers. Polymers are made from monomers or single units. A classic example is polyethylene. It is made from several monomer units of ethylene (CH2=CH2), an alkene, that combine through a process called polymerization to create polyethylene. The overall chemical reaction is as shown:
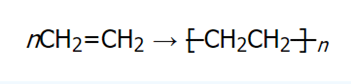
(Chemistry LibreTexts, 2020)
This same method of polymerization is used for other organic compounds to create various polymers used in plastics. Recycling codes are used for sorting the various types of plastics (or polymers) based mainly on their densities. Below is a table with the list of recycling codes and their associated polymers:
| Code | Acronym | Name of Polymer |
| 1 | PET | Polyethylene terephthalate |
| 2 | HDPE | High density polyethylene |
| 3 | PVC | Polyvinyl chloride |
| 4 | LDPE | Low density polyethylene |
| 5 | PP | Polypropylene |
| 6 | PS | Polystyrene |
| 7 | Other | Various |
This laboratory exercise will explore various plastics found in your household so that you can become more aware of the types of polymers that make up the plastics, their uses and applications in society.
Purpose
To identify, sort and gather information on plastics in your household to gain knowledge of recycling codes, their uses and applications.
Materials & Resources
Various household plastic bottles or containers
Future uses of plastics via https://plasticactioncentre.ca/directory/plastic-by-the-numbers/
Polymers information via https://chem.libretexts.org/Courses/Georgian_College/Chemistry_-_Academic_and_Career_Preparation/06%3A_Organic_Chemistry/6.13%3A_Polymers
Introductory video on plastics:
https://www.acs.org/content/acs/en/education/resources/undergraduate/chemistryincontext/interactives/world-of-polymers-and-plastics/chapter-opening.html
Video on recycling plastics:
https://www.acs.org/content/acs/en/pressroom/reactions/videos/2018/how-plastic-recycling-actually-works.html
Procedure
Part A
- Gather several plastic bottles or containers that you have available. Either ready to go to the recycling bin or in a cupboard.
- Look at the bottom of each type of bottle and try to find as many different recycling codes as possible. Fill in the table within the results sections to list the types of bottles or containers and their associated codes. Be specific, for instance, a ketchup bottle would correspond to recycling code 1. If you cannot find a specific recycling code plastic or container, please use the web and research which types of containers match the code you are looking for.
- Complete the remaining components within the results table using various resources available on the web. Resources are included in the Materials & Resources section above.
Part B
- Cut small pieces of the various recycling codes. One piece per code (if available).
- For each code, place the plastic piece into a cup of water. Indicate whether or not it sinks or floats using table 2. This tells us if it is more or less dense than water. Density of water is 1000 kg/m3.
- Answer the discussion questions that are related to both Part A and B of this experiment.
Results (34 marks)
Table 1. The types of plastic bottles or containers and their recycling codes.
| Recycling Code | Name | Types of bottles or containers found in your home | Monomer Unit | Future Use of this Plastic |
| 1 | PET | |||
| 2 | HDPE | |||
| 3 | PVC | |||
| 4 | LDPE | |||
| 5 | PP | |||
| 6 | PS | |||
| 7 | Other |
Table 2. Densities of the various plastics (14 Marks)
| Recycling Code | Name | Sink or Float | More or less dense than water |
| 1 | PET | ||
| 2 | HDPE | ||
| 3 | PVC | ||
| 4 | LDPE | ||
| 5 | PP | ||
| 6 | PS | ||
| 7 | Other |
Discussion Questions (13 marks)
- What is the most abundant type of plastic in your home? (1 mark)
- What type of plastic can be recycled and made into polyester, a clothing fabric? (1 mark)
- What type(s) of plastic could be used towards making a floatation device? What evidence do you have from the experiment to support your decision? (2 mark)
- Draw or take a snap shot image of the monomer unit used in creating PVC, PP and PS. Be sure to label the image with its appropriate monomer name (6 marks)
- Starch is considered a natural polymer. What is the monomer unit for starch? Provide two other examples of natural polymers (3 marks).
References
Chem LibreTexts (2020). 6.3 Polymers.
Do you need urgent help with this or a similar assignment? We got you. Simply place your order and leave the rest to our experts.
